Which Phrase Best Defines a Redox Reaction
Here is the word equation for the reaction. A reduction involves gaining electrons while an oxidation involves losing electrons.
Solved Thxidation And Reduction What Happens When Electrons Chegg Com
More Mnemonics for Redox.
. Yes it does because hydrogen H undergoes reduction and silver Ag undergoes oxidation. The formation of hydrogen fluoride is an example of a redox reaction. This is a redox reaction in which octane C8H18 is oxidized.
In a redox reaction an electron is lost by the reducing agent. The following equations are half reactions and reduction potentials. Which phrase best defines a galvanic cell.
Nuclear reactions produce more energy than conventional reactions. Yes it does because hydrogen H undergoes reduction and silver Ag undergoes oxidation. One species is oxidized and loses electrons which.
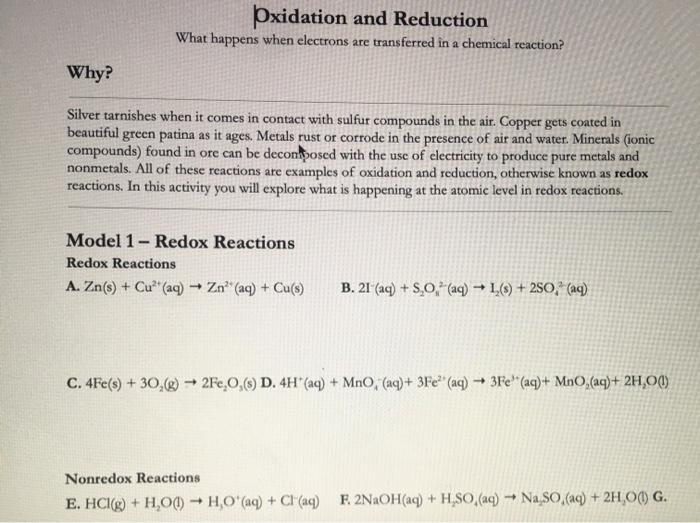
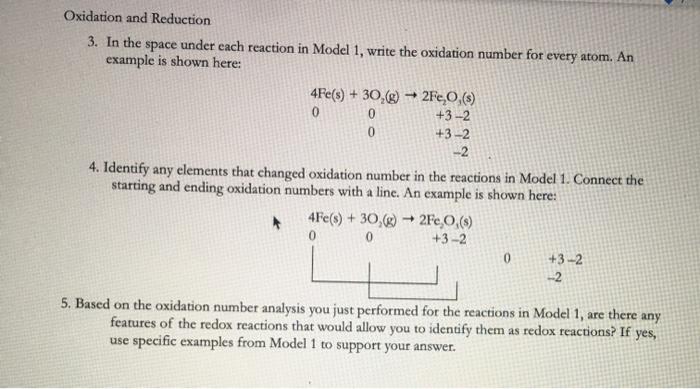
The key to identifying oxidation-reduction reactions is recognizing when a chemical reaction leads to a change in the oxidation number of one or more atoms. Which phrase best defines a redox reaction. The Loss of Electrons is Oxidation.
A reaction in which the exchange of electrons or oxygen occurs. O a reaction in which electrons are released from the system. Which product forms when two or more nonmetal atoms share valence electrons.
3 Which product forms two or more no metals atoms share valence electrons. The correct answer was given. An object that converts stored chemical energy into electrical energy an object that makes electrical connections an object that oxidizes atoms to produce ions an object that produces electrical energy through redox reactions.
It is worthwhile to re-memorize the rules. 11 The greatest amount of energy released per gram of reactants occurs during a. An object that produces electrical energy through redox reaction.
O a reaction in which the number of oxygen atoms is reduced. A Redox reaction involves the oxidation and reduction of the elements. If it is a redox reaction the oxidation numbers of two elements change from the reactant side to the product side.
2Ag sH2S g Ag2S sH2 g. It is nothing more than a bookkeeping system used to keep track of electrons in chemical reactions. The Gain of Electrons is Reduction.
O a reaction in which electrons are transferred between different atoms. An object that produces electrical energy through redox reactions. To keep the two halves of a redox process straight.
A chemical reaction involving the transfer of electrons from one math Analyze the pieces of the chemical reaction. Which action defines a double-displacement reaction. The term redox is a short form of reduction-oxidation.
Cr3 aq 3e- - Crs has a reduction potential of -074 V. 16 grams CH4 44 grams CO2 64 grams O2 36 grams H2O Organize the reactants what was present before the reaction and the products what is present after the. To find chemical reactions that are redox compare the oxidation numbers from the reactant side to the product side.
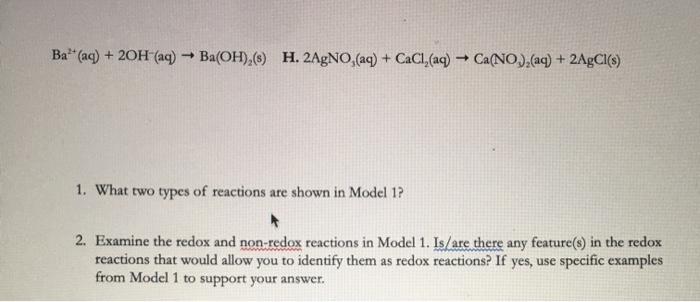
An oxidation-reduction redox reaction is a type of chemical reaction that involves a transfer of electrons between two species. The presence of which reactant is the best indicator of an oxidation-reduction reaction. Formation of a precipitate.
Which phrase is the best description of a redox reaction. In a redox reaction electrons are transferred from one species to another. Which of the following is true about a redox reaction.
Ag aq e- - Ags has a reduction potential of 080 V. Which answer best describes what is happening in the following reaction. Which statement best compares the substances in these half reactions.
Redox reactions are oxidation-reduction chemical reactions in which the reactants undergo a change in their oxidation states. Does this equation represent a redox reaction. A redox reaction an ionic compound a covalent compound 4 Which phrase best defines a galvanic cell.
A redox reaction is a chemical reaction that involves the transfer of electrons between chemical species. An oxidation-reduction reaction is any chemical reaction in which the oxidation number of a molecule atom or ion changes by gaining or losing an electron. You have probably learned the concept of oxidation number.
4 Which phrase best defines a galvanic cell. Column A Column b 2Which phrase best describes a redox reaction. Yes it does because H undergoes reduction and zag undergoes oxidation.
An object that produces electrical energy through redox reactions An object that makes electrical connections An object that converts stored chemical energy into electrical energy. An object that produces electrical energy through redox reactions. Why does the reaction H2OAgno reaction not proceed to a product.
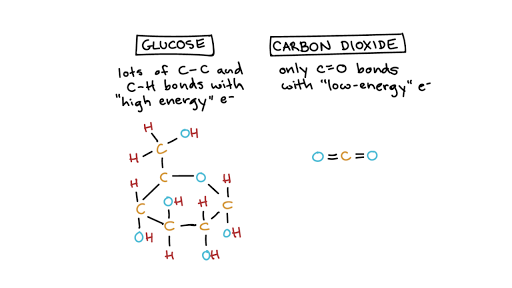
Iron water oxygen hydrated ironIII oxide Iron and steel rust when they come into contact with water and oxygen. 1 point O a reaction in which oxygen combines with different atoms. All the redox reactions can be broken down into two different processes a reduction process and an oxidation process.

How To Say The Food Was Very Good La Comida Estaba Muy Rica In Span Good In Spanish Spanish Language Learning Learning Spanish

Naming Ionic And Molecular Compounds How To Pass Chemistry Youtube Chemistry Molecular Science Chemistry
Solved Thxidation And Reduction What Happens When Electrons Chegg Com

Typography And Quotes Quotes Words Quotes Inspirational Quotes

No Chemicals No Problem Use Gatorade And Drain Cleaner For An Easy Color Change Chemistry Demonstration Dif Chemical Changes Chemical Kinetics Drain Cleaner

Balancing Redox Equations Article Khan Academy

6 5 Classifying Chemical Reactions Redox Chemistry Libretexts

Introduction To Cellular Respiration And Redox Article Khan Academy

Oxidation Reduction Redox Reactions Article Khan Academy

Balancing Redox Equations Article Khan Academy

Top Best Barry Melrose Quotes Inspiration And Motivation With Photos 2020 In 2021 Some Motivational Quotes Quotes Inspirational Quotes

Oxidation Reduction Redox Reactions Article Khan Academy
Solved Thxidation And Reduction What Happens When Electrons Chegg Com

Guided Reading 6 Electrochem Part1 Week 6 Electrochemistry Week 6 Chemical Reactions 4 Studocu

Solved Indicate Whether The Statement Is True Or False If False Change The Identified Word Or Phrase To Make The Statement True 7 Marks 11 The Algebraic Sum Of The Positive And Negative

Understanding Redox Reactions Oxidation Reduction Chemtalk

Solved Question 1 In The Redox Reaction Shown Below Ag Fe Zag Fe 20 3 Fe Is Enter The Word Oxidized Or Reduced In The Space Provided Que Stion 2 In The


Comments
Post a Comment